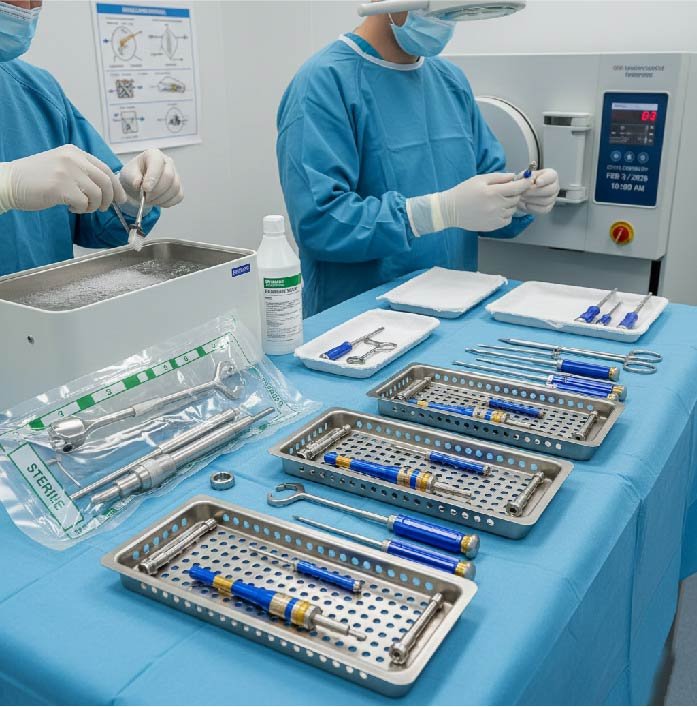
Introduction to Cannulated Screw Instrument Sets
Sets of cannulated screws are the unspoken heroes of the modern field of orthopedic surgery. They are in the background, assisting surgeons to achieve precise fixation using minimum invasiveness. But here’s the thing, the complexity of their design makes them difficult to clean, sterilizing and maintain. In the event of a mistake, you’re not just putting yourself at risk with the instrument but also the safety of patients.
This article delved into the maintenance and sterilization of sets of cannulated screw instruments by breaking down complicated processes into easy-to-follow steps. Imagine it as an outline to ensure that these instruments are secure, efficient and durable.
What Are Cannulated Screw Instrument Sets?
Sets of screw instruments that cannulate comprise guide wires, drills and taps, as well as the depth gauge, screws and screws – all designed to work with hollow channels. These cannulations permit exact placement over guide wires, which is great for performing surgery, but difficult to use to reprocess.
Why Proper Sterilization and Maintenance Matter
Why is all the fuss? Because instruments that are cannulated can hold blood tissues, bone, and blood in their lumens. If they’re not properly cleaned, these hidden pollutants can be able to survive sterilization cycles and lead to infection, delays in surgery or failure of the instrument. In short, proper maintenance isn’t an option; it’s mandatory.
Understanding the Complexity of Cannulated Instruments
Cannulated instruments don’t look like blunt forceps or scissors that are flat. They’re more like tunnels. Tunnels love to conceal things.
Design Features That Require Special Care
The long, narrow lumens, as well as the inner threads, can make washing a challenge. Simple rinsing isn’t enough.
Cannulations, Lumens, and Hidden Surfaces
If you aren’t able to be able to see it, you have to wash it. This is an absolute rule. Cannulations require specific brushes, adapters, and flushing systems to access every surface inside.
Risks of Improper Sterilization
The idea of cutting corners here is similar to driving without brakes in a car – dangerous and ineffective.
Infection Control and Patient Safety
Residual bioburden could cause osteomyelitis, infection at the site of surgery and long healing times. This is a huge cost for not cleaning properly.
Instrument Damage and Reduced Lifespan
The rust and dried debris slowly destroy instruments, leading to distortion, stiffness, and ultimately failure.
Regulatory Standards and Guidelines
Sterilization isn’t guesswork; strict regulations guide it.
International Sterilization Standards
Organizations such as ISO, AAMI, and CDC have clear guidelines for cleaning, disinfection and sterilization for surgical equipment.
Manufacturer Instructions for Use (IFUs)
Always adhere to the IFU. It’s not just a piece of advice, it’s an obligation. Different alloys, coatings and designs require specific processes for reprocessing.
Pre-Cleaning at Point of Use
The process of cleaning begins at the time of operation.
Immediate Post-Procedure Care
Cleanse instruments after surgery and flush cannulations with clean water as soon as you have finished using them.
Preventing Drying of Bioburden
After it dries, it’s similar to concrete. Keeping instruments moist makes cleaning far easier later.
Manual Cleaning of Cannulated Screw Instruments
The manual cleaning process is at the foundation of correct Reprocessing.
Step-by-Step Manual Cleaning Process
Disassemble the instruments, then soak them in an enzyme-based detergent, then scrub the surfaces thoroughly, and thoroughly flush cannulations.
Importance of Brushing Cannulations
Make sure you use the right size brush. Too small brushes will not clean, and too big ones could damage the lumen.
Ultrasonic Cleaning for Cannulated Sets
Imagine ultrasonic cleaners as power washers for surgical instruments.
How Ultrasonic Cleaning Works
High-frequency sound waves cause tiny bubbles that explode and dislodge debris from difficult-to-access regions.
Best Practices for Cannulated Instruments
Utilize lumen adapters to ensure that the solution to clean is flowing through cannulations, not just around them.
Automated Washer-Disinfectors
Automatization can be reliable, but it’s not foolproof.
Advantages of Automated Cleaning
They minimize the chance of human error as well as standardize processes.
Limitations of Cannulated Designs
If they don’t have the right adapters, washer-disinfectors might not be able to clean internal channels efficiently.
Inspection and Functional Testing
If you don’t check the area, you aren’t aware.
Visual Inspection Techniques
Make use of magnification and adequate lighting to examine surfaces as well as joints, tips and surfaces.
Checking Cannulations for Debris
Borescopes have the potential to revolutionize this that allows for visual inspection of the lumens.
Packaging and Assembly for Sterilization
Packaging is more important than you think.
Proper Tray Configuration
Avoid stacking instruments. The overcrowding of instruments can block the sterilant flow.
Ensuring Sterilant Penetration
Open hinges to allow for vertical alignment of cannulations when feasible, and use certified containers or wraps.
Sterilization Methods for Cannulated Screw Sets
Different situations require different strategies.
Steam Sterilization
It is the gold standard. Dependable, effective and widely accessible when employed correctly.
Low-Temperature Sterilization Options
Hydrogen peroxide plasma, also known as the EO gas, is a possibility for components that require heat.
Storage and Handling After Sterilization
Sterility doesn’t cease when you get an autoclave.
Maintaining Sterile Integrity
Be careful when handling trays. A tear in the wrap could ruin all the work you’ve done.
Environmental Considerations
Clean, dry, and temperature-controlled storage areas are non-negotiable.
Routine Maintenance and Lubrication
Maintenance is a preventive treatment for instruments.
Instrument Lubricants and Their Role
Make use of lubricants made of steam and water to safeguard moving components.
Preventing Corrosion and Wear
Dry the instruments thoroughly and stay clear of harsh chemicals that can damage the protective coatings.
Common Mistakes to Avoid
Let’s put these in plain English.
Skipping Cannulation Cleaning
This is probably the most well-known and most dangerous mistake.
Overloading Trays
More instruments in a tray might appear efficient, but it can compromise the cleaning process and the sterilization.
Staff Training and Documentation
People are as important as the process.
Importance of Skilled Personnel
Regular training keeps employees up-to-date on the best practices and latest technologies.
Record-Keeping and Traceability
Documentation is a way to ensure accountability and can be helpful during audits or investigations into incidents.
Future Trends in Cannulated Instrument Care
The future will be smarter and cleaner.
Innovations in Instrument Design
Manufacturers are working on instruments that have cleaner cannulations and more modular designs.
Advanced Cleaning Technologies
Automated systems for flushing lumens and inspection tools that use AI are on the horizon.
Conclusion
Maintenance and sterilization of sets of cannulated screw instruments aren’t the most glamorous of tasks; however, they are vital. These instruments require a lot of attention because of their intricate design. When properly handled, they will provide you with security, reliability and long-term use. Consider proper reprocessing as an investment, not just in instruments, but also in results for patients and surgical outcomes. Be sure to do it right each time, and everyone wins.
Frequently Asked Questions
- Are cannulated instruments more difficult to clean than instruments made of solid materials?
It’s because their hollow lumens hold in dirt that conventional cleaning methods aren’t able to get in.
- Does ultrasonic cleaning have to be a requirement for screw sets with cannulated screws?
Although it is not required in all cases, it is highly recommended for efficient internal cleaning.
- Can steam sterilization cause damage to cannulated instruments?
It’s not if the manufacturer’s guidelines are adhered to and the instruments are maintained properly.
- How often should instruments that have cannulas be checked?
After each cleaning cycle, and before packaging, to sterilize.
- What is the greatest danger of poor cleaning?
Infections at the surgical site and compromised safety of patients.